Basic QC Practices
Another look at Pre-Analytical Error Rates, 2021
Our review of several publications on pre-analytical quality finds that the convergence of world pre-analytical quality is upon us. That, and the plateau is real.
2021 Review of Pre-Analytical Error Rates
Convergence of World Practice and the Further Plateau of Progress
Sten Westgard, MS
March 2021
Every few years we like to revisit the issue of pre-analytical quality in the laboratory. We don't spend a lot of time on it here, mostly because this subject is thoroughly, exhaustively covered almost everywhere else. There are entire conferences devoted to pre-analytical qualty. Standing committees exist - some have existed for decades now - devoted to the improvement and eradication of errors in the pre-analytical phase.
Since the early part of the effort on pre-analytical effort, huge progress has been made in improving, systematizing, and automating pre-analytical processes. The whole taxonomy of pre-analytical errors has been established and efforts are being made to regularly tracking those rates. The EFLM Pre-analytical working group has also converted those measures into Sigma-metrics.
A few years ago, we began to see a plateau of progress. The early gains are over, now not only will we make fewer gains, but it will take a lot more effort. When the curve flattens out, you begin to evaluate whether the same amount of effort can be better applied (with more return) to other areas of the total testing process.
The latest series of papers confirms this plateau:
- Pre-analytical quality indicators in laboratory medicine: Performance of laboratories participating in the IFCC working group "Laboratory Errors and Patient Safety" project. Laura Sciacovelli, Giuseppi Lippi, Zorica Sumarac et. al. Clinical Chimca Acta 497 (2019) 35-40.
- Evaluation of quality indicators in pre-analytical phase of testing in clinical biochemistry laboratory of a tertiary care hospital in India. Nishtha Wadhwa. International Journal of Clinical Biochemistry and Research 2020 ;7(3):354-356.
- Brazilian laboratory indicators benchmarking program: three-year experience on pre-analytical quality indicators. Wilson Shcolnik, Fernando Berlitz, Cesar Alex de O. Galoro, et. al. Diagnosis 2020 Aug 31;dx-2020-0043.
- Three year's experience of quality monitoring program on pre-analytical errors in China. Fengfeng Kang, Weixing Li, Xiaohua Xia, Zhiming Shan. J Clin Lab Anal. 2021 Mar;35(3):e23699.
Here we have surveys from Brazil, China, and the IFCC program, as well as a single hospital lab in India. Let's throw them all together and put them on the Sigma Scale:
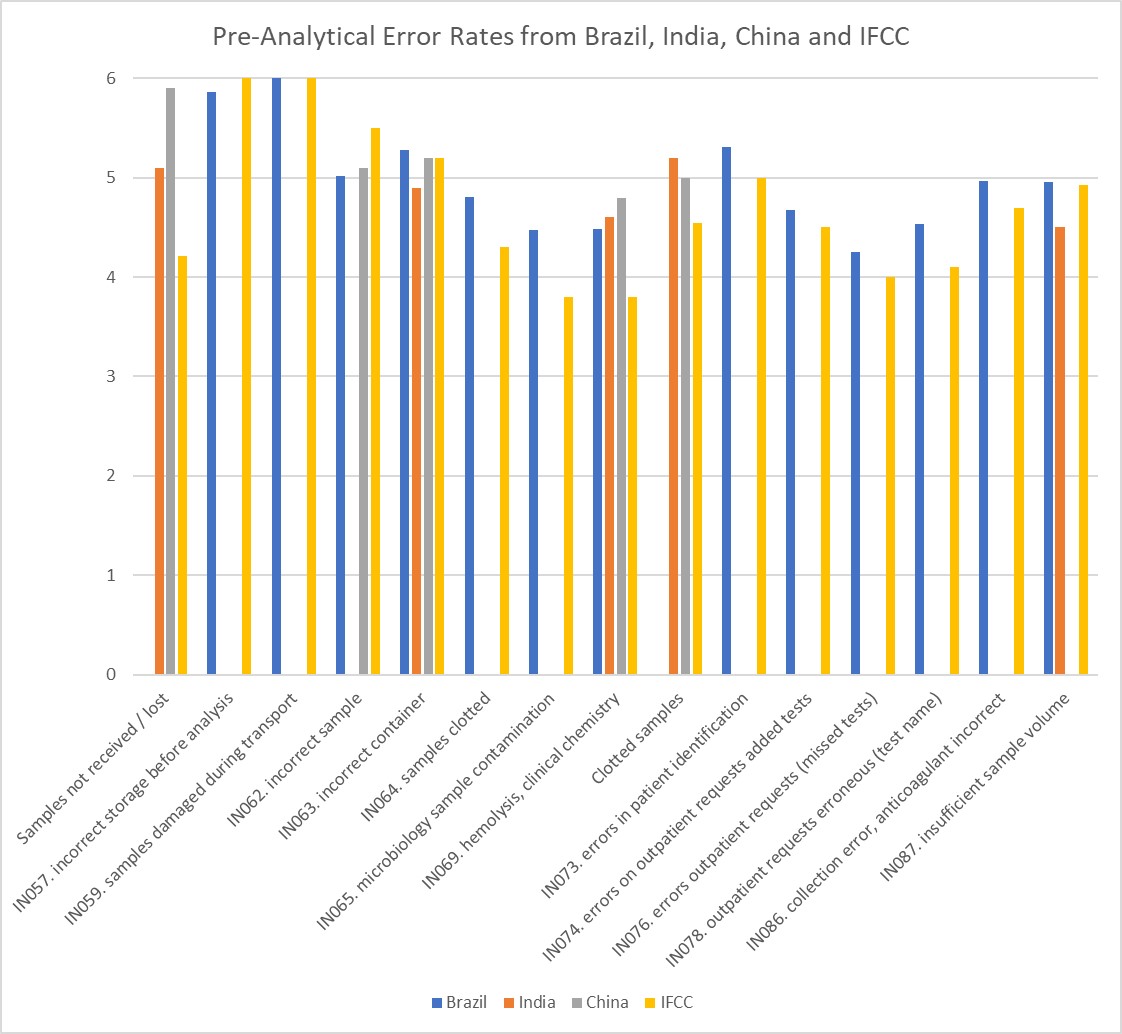
This is a bit hard to digest, I know. There are many indicators here, some referred to by number (from the Brazilian program). But the key takeaway is to see the "band" of where these Sigma metrics are located. Everything is pretty much 4 Sigma and higher. There are only a few indicators that all the studies cover, but if you look at the "incorrect container" indicator, that is very stable across all the studies. The metric seems to be at around 5 Sigma. Remember, 4 Sigma is good, 5 Sigma is excellent, 6 Sigma is world class. When the first Sigma metrics were reported by Nevelainen et. al. in 2001, 20 years ago, there was nothing close to 6 Sigma. Now, we have reached a point where 6 Sigma is possible and within reach of many labs (for some processes).
Now let's look at the Brazilian program, where they measured quality in three successive years, 2016, 2017, 2018:
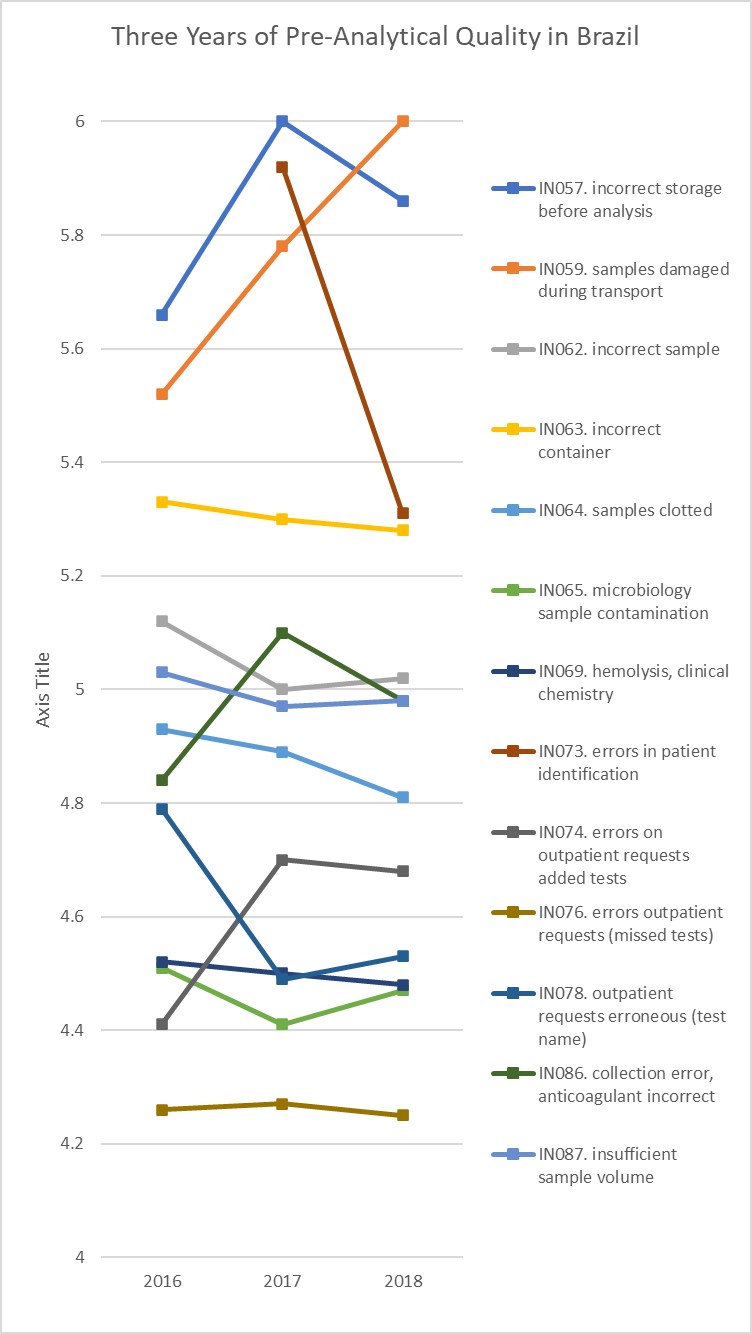
In this case, we've narrowed the y-axis to the 4 Sigma and up zone. Notice how many indicators are "flat" - they have essentially not changed in 3 years. Granted, there are still a few indicators where there is some fluctuation, but our y-axis focus accentuates that fluctation. The most change we see is about a 0.5 Sigma-metric shift in the Patient Identification indicator, which remains before and after in the 5 Sigma zone, a change of only 66 defects per Million (from 5 DPM to 72 DPM), or a change of 0.00654 %. We're chasing the very thin part of the wedge when we focus on that process.
Here's another three-year check, this time from China:

Here the quality is very flat - sometimes so flat that a line will disappear behind another line.
Let's be clear, pre-analytical errors are important. We should monitor them, we should improve them, we should not relax our vigilance and let them creep up again.
However, if we come across much larger error rates in the analytical phase (and we do, we do), we should no longer dismiss them because "errors are only significant in the pre-analytical phase." That argument no longer holds water. The new rates of pre-analytical errors show that success has made them rare.
Analytical errors are the new largest error we need to tackle.
