Basic QC Practices
A look at pre-analytical error rates, 2018
The continuing discussion of pre-analytical quality in the literature prompts us to update our review of current laboratory defect rates. From a laboratory in India, what can we see in recent developments in processes
Another look at Laboratory Error Rates, India 2018
Sten Westgard, MS
APRIL 2018
Evaluation of Preanalytical Quality Indicators by Six Sigma and Pareto's Principle, Sweta Kulkarni, R. Ramesh, A.R. Srinivasan, C.R. Wilma Delphine Silvia Ind J Clin Biochem (Jan-Mar 2018) 33(1): 102-107.
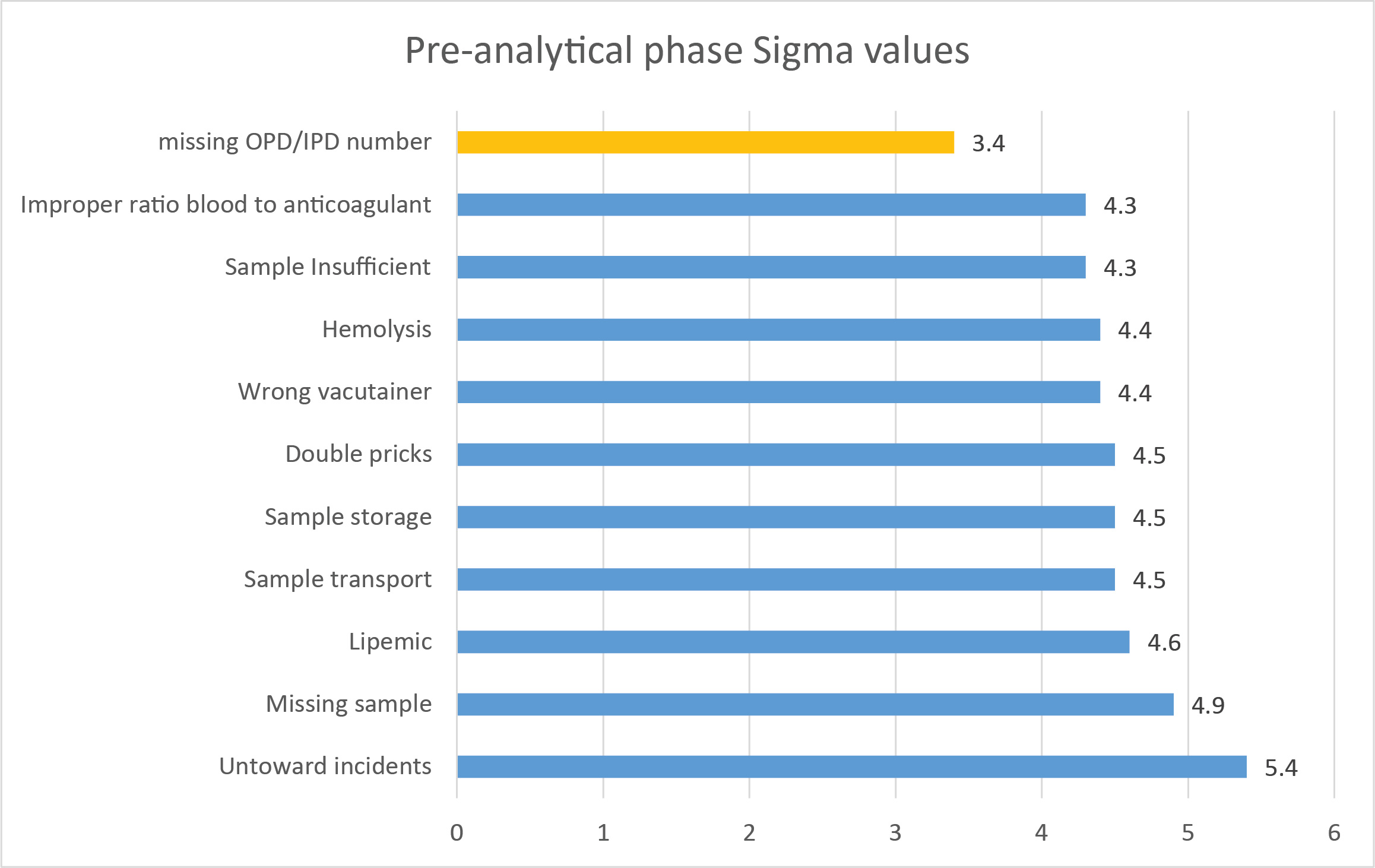
Notice how most of these processes are above 4 Sigma, which is considered good on the Six Sigma scale. The lone low-Sigma number was the failure to have the correct patient identifier associated with the vacutainer.
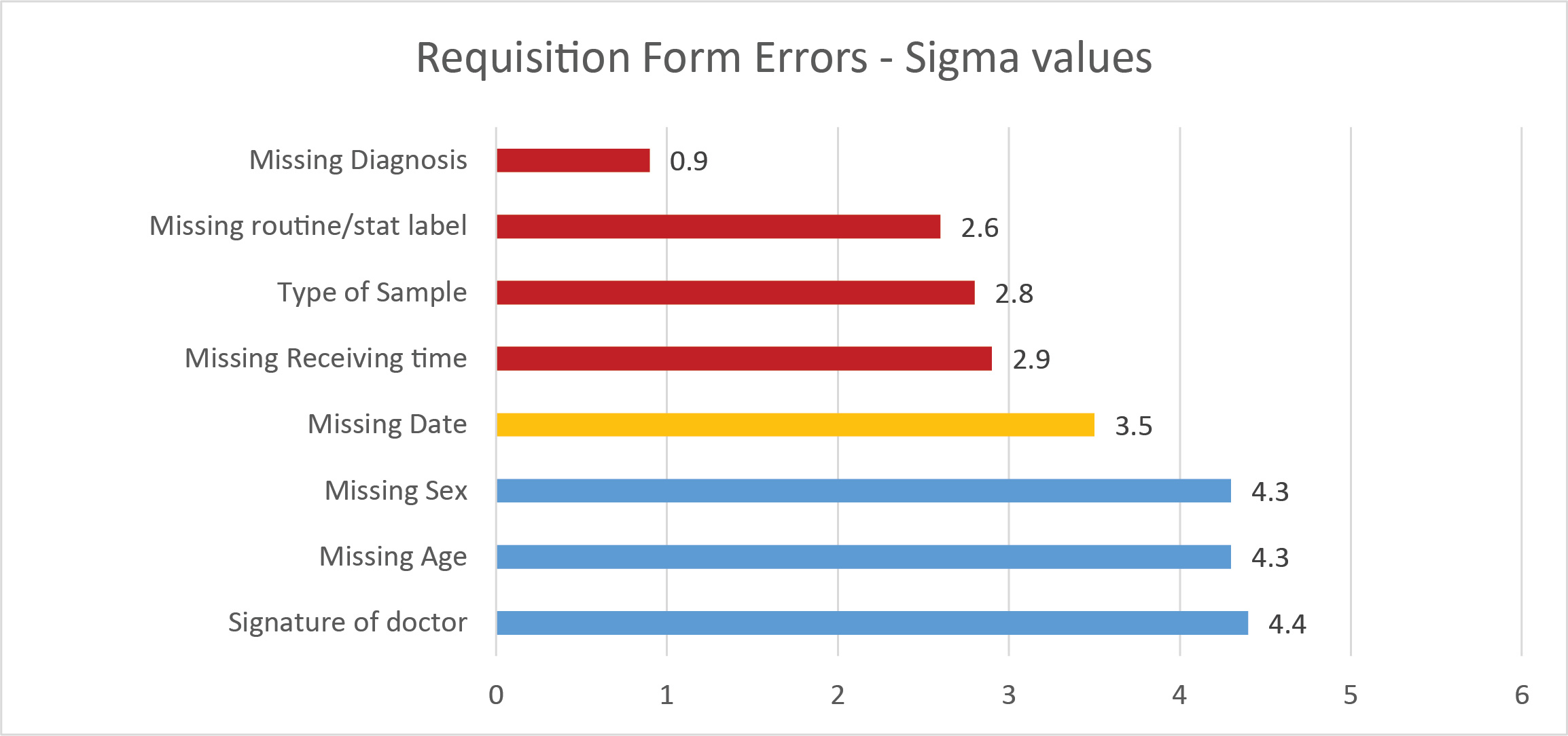
Where the Pareto Principle comes in for this study is the finding that 80% of the errors occurred in the filling out of the requisition form, while only 20% of the errors were sample collection.
The authors concluded "Although the test requisition forms are filled in by interns and doctors there is a lack of awareness on [the] importance of filling the information in forms. The diagnosis or brief note on patient's symptoms helps the clinical biochemist to interpret the report and this information was lacking in most of the forms."
What is not quite so clear here is how much higher many of these pre-analytical processes are than what we see on the analytical side. More analytical tests may be found to have much lower Sigma-metrics than what's happening on the pre-analytical side.
While pre-analytical processes are crucial, as we see global improvements to their sigma-metrics, this means pre-analytical errors are less and less likely to be the biggest problem in the laboratory.
