Basic Planning for Quality
QP 3: Complying with Regulations, Standards, and Practice Guidelines
JCAHO, CLIA, NCCLS - which guidelines define quality in the lab? And which rules and requirements trump the other rules? Dr. Westgard sorts through all the different regulations, recommendations, and emerges with the critical directions. (Preview)
- JCAHO Guidelines for Improving Organizational Performance
- CLIA rules and Regulations
- NCCLS QC Practice Guidelines
- Other Guidelines
- References
Implementation of a quality planning process should be a high priority in laboratories today to assure or guarantee the quality of laboratory tests and services. Guidelines for quality planning can be found in government regulations, accreditation standards, and national practice standards. The widespread application of quality planning is recommended in the latest inspection manual from the Joint Commission for Accreditation of Healthcare Organizations (JCAHO). A focus on analytical quality, the assessment of method performance, and the selection of appropriate quality control procedures is provided by the Clinical Laboratory Improvement Amendments (CLIA-88). A general planning methodology for quality control is outlined by the National Committee for Clinical Laboratory Standards (NCCLS). Together, these guidelines provide the basis for implementing a quality planning process in laboratories. Here's a summary of the standards, rules, and recommendations that bear on quality planning.
JCAHO Guidelines for Improving Organizational Effectiveness
Improving Organizational Performance, or IOP, is JCAHO's latest terminology and represents an evolution of their TQM philosophy and concepts. The 2000-2001 Comprehensive Accreditation Manual for Pathology and Clinical Laboratory Services [1] states that the goal of IOP is "for the laboratory to design processes well and systematically monitor, analyze, and improve its services that affect patient health outcomes." The following are identified as essential management activities:
- Designing processes;
- Monitoring performance through data collection;
- Analyzing current performance; and
- Improving and sustaining improved performance.
Review of specific standards
More guidance is provided by specific standards. In the list that follows, I have also identified how these standards fit into the total quality management framework described earlier, which is composed of quality laboratory practices (QLP), quality control (QC), quality assessment (QA), quality improvement (QI), quality planning (QP), and quality goals (QG). This additional information will be helpful for assessing the relationship between IOP and TQM.
- PI.1 The leaders establish a planned, systematic, organization-wide approach to process design and performance measurement, analysis, and improvement. [QP to establish QLP]
- PI.1.1 The activities are planned in a collaborative and interdisciplinary manner. [QP across functions or departments to establish QLP]
- PI.1.2 New or modified processes are designed well. [QP or re-planning to establish QLP]
- PI.2.1 Performance expectations are established for new and modified processes. [QG or requirements for QLP]
- PI.2.2 The performance of new and modified processes is measured. [QA of QLP]
- PI.3 Data are collected to monitor the stability of existing processes, identify opportunities for improvement, identify changes that will lead to improvement, and sustain improvement. [QC and QA of QLP]
- PI.3.1 The organization collects data to monitor its performance. [QA]
- PI.3.1.1 The organization collects data to monitor the performance of processes that involve risks or may result in sentinel events. [QA of QLP]
- PI.3.1.2 The organization collects data to monitor performance in areas targeted for further study. [QA]
- PI.3.1.3 The organization collects data to monitor improvements in performance. [Q A]
- PI.4 Data are systematically aggregated and analyzed on an ongoing basis. [QA]
- PI.4.1 Appropriate statistical techniques are used to analyze and display data. [QA and QI]
- PI.4.2 The organization compares it performance over time and with other sources of information. [QA and QI]
- PI.4.3 Undersirable patterns or trends in performance and sentinel events are intensively analyzed. [QA and QI]
- PI.4.4 The organization identifies changes that will lead to improved performance and reduce the risk of sentinel events. [QI and QP or re-planning]
- PI.5 Improved performance is achieved and sustained. [QP, QLP, QC, QA, QI]
IOP vs TQM
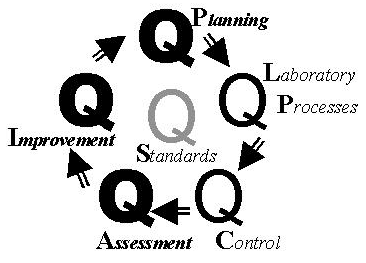 From the review of these standards and comparison to our TQM framework, the relationship between IOP and TQM is described in the accompanying figure, where theemphasis on each component is shown by the darkness of each Q. Quality Planning is strongly recommended by the JCAHO standards on the "design" of processes. Quality Laboratory Processes is pervasive throughout the standards and JCAHO's frequent reference to "processes." Quality Control and Quality Assessment provide the measure and monitor aspects that are mentioned in a large number of the standards. Quality Improvement is the focus of the whole IOP approach and is specifically mentioned in several standards. Quality goals are found in the standard that recommends establishing performance expectations for new and modified processes.
From the review of these standards and comparison to our TQM framework, the relationship between IOP and TQM is described in the accompanying figure, where theemphasis on each component is shown by the darkness of each Q. Quality Planning is strongly recommended by the JCAHO standards on the "design" of processes. Quality Laboratory Processes is pervasive throughout the standards and JCAHO's frequent reference to "processes." Quality Control and Quality Assessment provide the measure and monitor aspects that are mentioned in a large number of the standards. Quality Improvement is the focus of the whole IOP approach and is specifically mentioned in several standards. Quality goals are found in the standard that recommends establishing performance expectations for new and modified processes.
In summary, JCAHO provides a broad recommendation for quality planning that applies to all laboratory processes. Given that the fundamental laboratory process is to perform tests, carefully planned testing processes should be the heart of quality laboratory processes. Performing tests is the main event in the laboratory. Other activities are important in support of testing and patient services, but none of them willl matter if the laboratory can't produce correct test results.

 We invite you to read the rest of this article
We invite you to read the rest of this article
Note: This material is covered in the Basic Planning for Quality manual, which is availalbe in our online store. You can download the Table of Contents and additional chapters here.
Updated and expanded coverage of these topics can be found in Assuring the Right Quality Right,also available in our online store. You can also download the Table of Contents and additional chapters here.
Finally, you can access materials online on these topics by enrolling in the Management and Design of Analytical Quality Systems course course.
