Basic Method Validation
Selecting a Method to Validate
Before you run all the experiments and crunch all those numbers and print out the report, make sure you've chosen the right method to validate. Methods are not one-size-fits-all when in comes to your laboratory. In this lesson, Dr. Westgard helps you choose the right method for your unique needs. (Preview)
| Note: This lesson is drawn from the first edition of the Basic Method Validation book. This reference manual is now in its fourth edition. The updated version of this material is also available in an online training program |
MV - Selecting a Method To Validate
- Establishing a laboratory testing process
- Method characteristics
- AST example from the literature
- Cholesterol example
- Method evaluation vs method validation
- References
Error assessment is what method validation is about, as discussed earlier in MV - The Inner, Hidden, Deeper, Secret Meaning. However, before getting to the assessment of errors, you have to first select the method to be validated. Method selection is a different process that needs to be understood in relation to the validation process that will follow. In fact, there are several other processes that are essential for establishing a routine method of analysis.
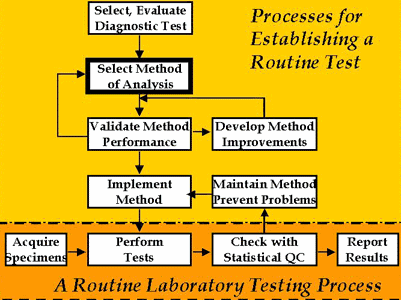 Establishing a laboratory testing process
Establishing a laboratory testing process
Important activities for establishing a routine method of analysis are shown in the accompanying figure. The blocks at the bottom illustrate the key steps involved in routine analysis, where the laboratory acquires specimens, performs tests, checks statistical QC, and reports test results. Those activities are generally regarded as the real work of the laboratory.
However, for analysis to become routine, the other activities shown in the figure are very important. The selection of the diagnostic test is actually the first step, but this is often skipped for common tests whose medical usefulness is well accepted. For these well accepted tests, we usually start with the selection of the method (the box with the darkest border), then validate its performance. If performance is acceptable, the method is implemented for routine service. If performance is not acceptable, the laboratory may develop some improvements, although that is becoming increasingly difficult with the high state of automation of many analytical systems. Today it's more likely that a laboratory would select another method rather than attempt to make improvements, then start the validation process over again for the new method.
Once a method is demonstrated to perform acceptably, the method must be implemented for routine operation. This involves defining the standard operating procedures and documenting the procedure, selecting an appropriate QC procedure for monitoring routine performance, and training personnel to operate the new method. While in routine service, problems will undoubtedly be identified through QC, which will lead to preventive maintenance procedures to minimize or eliminate those problems. Routine operation is often the simplest part of this overall process if the laboratory does a good job of selecting the method, validates method performance, implements the method through careful and thorough in-service training, monitors method performance with a QC procedure that has a low false rejection rate and appropriate error detection, and aggressively maintains the method to identify problems, eliminate sources of error, and prevent future problems from occurring.
We invite you to read the rest of this article.
This article, plus many more important, updated, and expanded chapters are available in the Basic Method Validation manual, 3rd Edition which is available at our online store. You can download the Table of Contents and other chapters here. You can also enroll in the Basic Method Validation course and access the new materials online.


