Sigma Verification of Performance Program
10th People's Hospital of Shanghai Verification of Sigma Performance
The 10th People's Hospital of Shanghai achieved Sigma Verification on October 25th, 2021.
The 10th People's Hospital of Shanghai Sigma Verification of Performance
Verification period: October 25th, 2021 through October 31st, 2022
"The Department of Laboratory Medicine, Shanghai Tenth People’s Hospital has been in operation for more than 70 years, and through the efforts of several generations, it has developed into a key department in the hospital with good instrumentation, highly qualified staff, comprehensive testing programs, and an annual testing workload of more than 17.1 million times, integrating clinical, teaching and scientific research. At present, the department has carried out more than 400 kinds of tests, and the gene amplification laboratory (PCR laboratory) is one of the first laboratory accredited by the State Ministry of Health in Shanghai. In 2010, we successfully passed the medical laboratory quality and competence accreditation (ISO 15189:2007). For many years, we have participated in the external quality assessment activities organized by the Clinical Laboratory Quality Control Center of the Ministry of Health and the Shanghai Clinical Laboratory Quality Control Center with excellent results. And achieved good results in global external quality assessment activities in several specialties. In recent years, the department has been focused on basic and clinical research with molecular diagnosis as its characteristic and precise diagnosis as its direction. In the past ten years, the team has been awarded 41 projects, including 2 projects of National Program on Key Basic Research Project, 24 projects of National Natural Science Foundation of China,1 project of the National Science Fund for Excellent Young Scholars, 1 project of National Natural Science Foundation of China(Key Program), 1 project of Shanghai Collaborative Innovation Cluster, 1 project of Shanghai Important Weak Discipline, and 2 projects of Shanghai New Excellent Youth.
The team has published more than 70 related papers in international academic journals such as Mol Cancer, Autophagy, Nucleic Acid Research, Nature Communication, Hepatology, etc. Focus on cross-disciplinary cooperation to combine molecular biology with emerging technologies such as nano-biosensing for the analysis of tumor-related markers such as micro RNA and circulating tumor cells. Backbone members of the team has published 61 papers in J. Am. Chem. Soc., ACS Nano and other international authoritative journals as first or corresponding author, including 15 papers with 10 or more impact factor."
The Analytes which Achieved Verification
|
Chemistry Assay |
AC02677 verified |
AC02678 verified |
AC02679 verified |
|
Albumin |
Six Sigma |
- |
Six Sigma |
|
Bilirubin, Direct |
- |
- |
Six Sigma |
|
Chloride |
Four Sigma |
- |
- |
|
Creatinine Kinase |
- |
Six Sigma |
- |
|
Creatinine |
- |
Four Sigma |
- |
|
Glucose |
Six Sigma |
- |
- |
|
LDH |
- |
Six Sigma |
- |
|
Phosphorous |
Six Sigma |
- |
- |
|
Potassium |
Six Sigma |
- |
- |
|
Total Protein |
- |
- |
Six Sigma |
|
Urea Nitrogen |
- |
Four Sigma |
- |
|
Uric Acid |
- |
Six Sigma |
- |
|
Chemistry Assay |
AC02680 verified |
AC02681 verified |
C1602106 verified |
|
Alkaline Phosphatase |
Six Sigma |
|
|
|
AST |
|
Four Sigma |
|
|
Bilirubin, Total |
|
Four Sigma |
|
|
Cholesterol |
Six Sigma |
|
|
|
GGT |
|
Six Sigma |
|
|
Iron |
|
|
Four Sigma |
|
Magnesium |
|
|
Five Sigma |
|
Triglycerides |
Six Sigma |
|
|
|
UIBC |
|
|
Six Sigma |
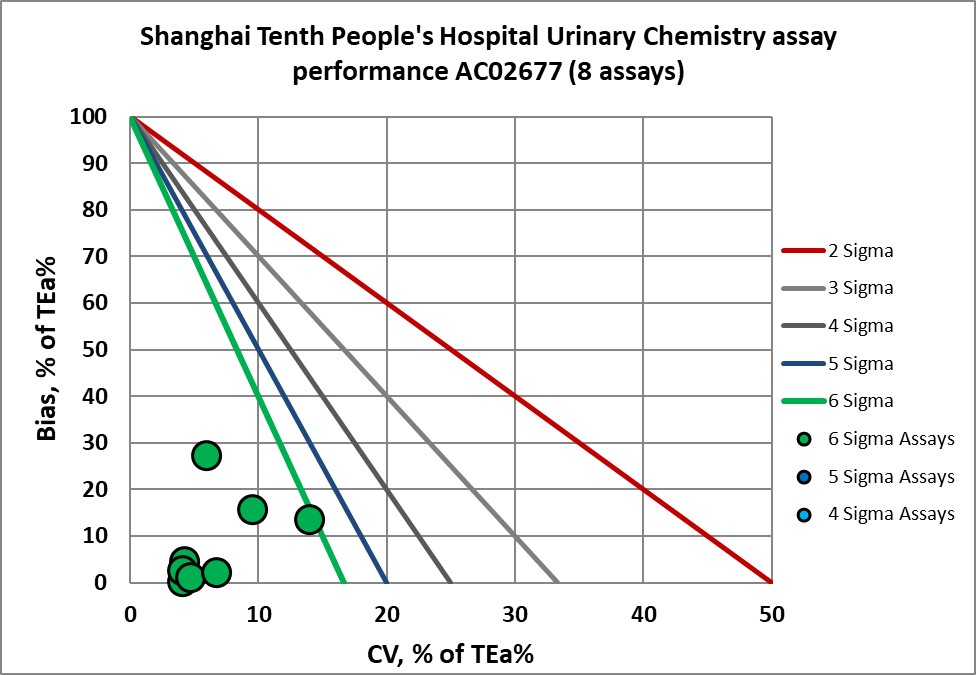
Urine assays were also verified:
|
Urinary Chemistry Assay |
AC02677 verified |
|
Calcium, U |
Six Sigma |
|
Chloride, U |
Six Sigma |
|
Creatinine, U |
Six Sigma |
|
Glucose, U |
Six Sigma |
|
Potassium, U |
Six Sigma |
|
Sodium, U |
Six Sigma |
|
Urea N, U |
Six Sigma |
|
Uric, U |
Six Sigma |
Graphic display of Sigma metric performance
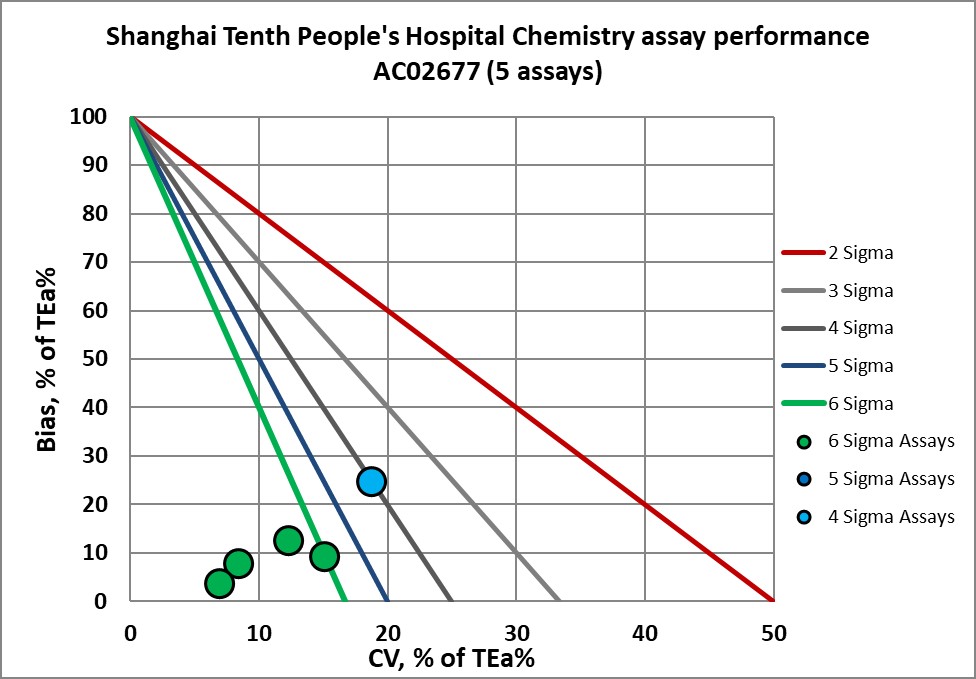

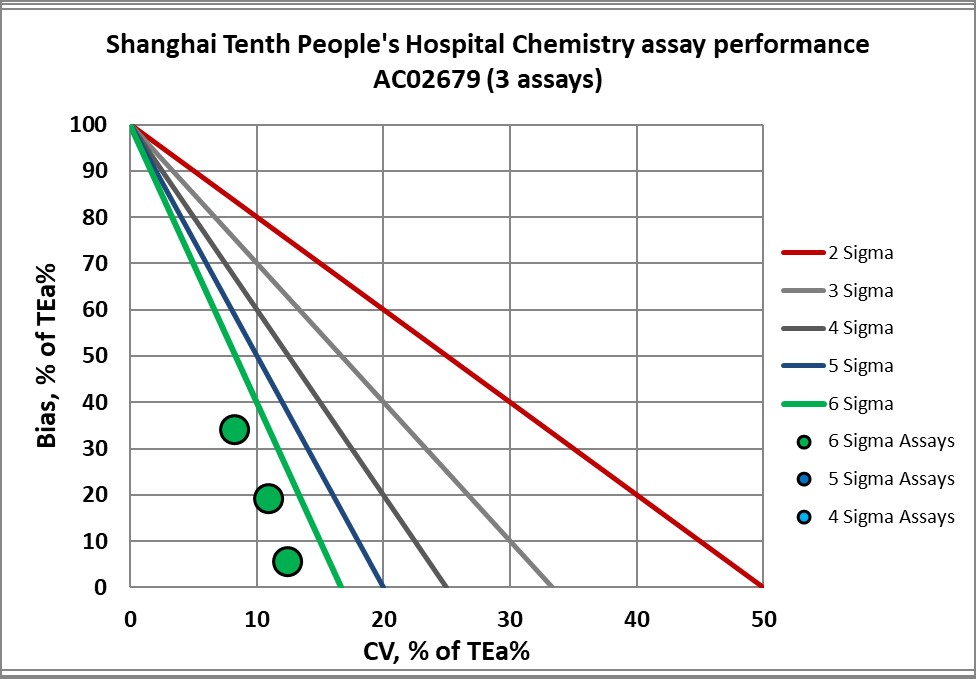
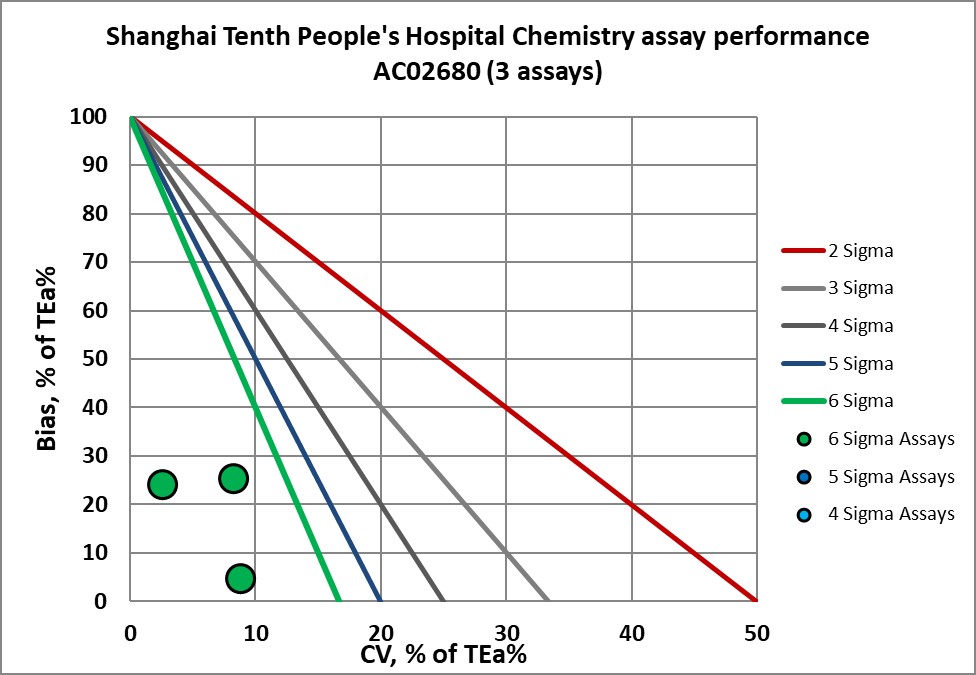

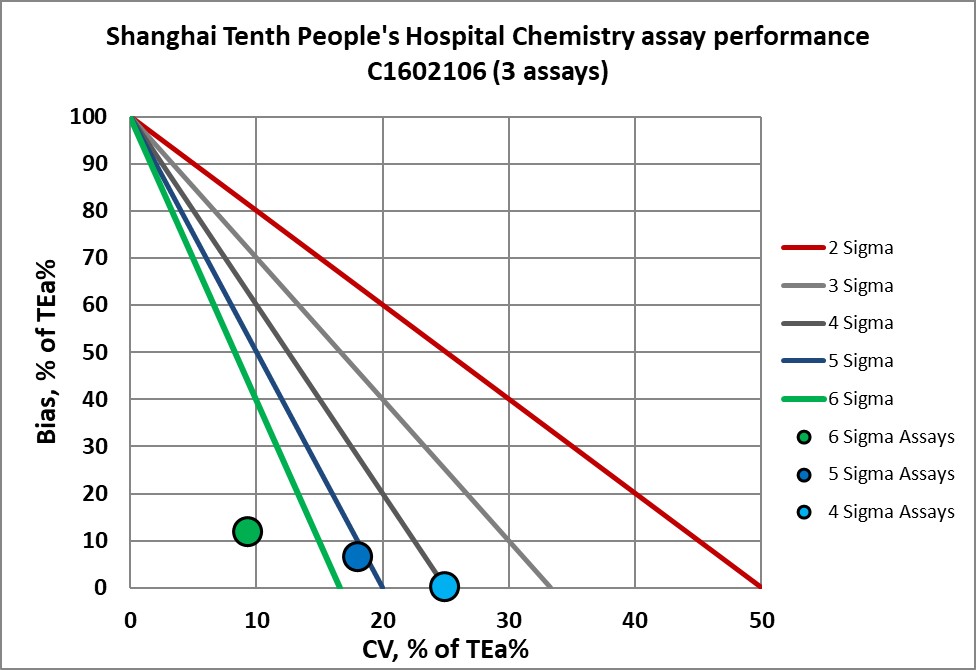
Congratulations to the lab and staff of Shanghai 10th People's Hospital.
